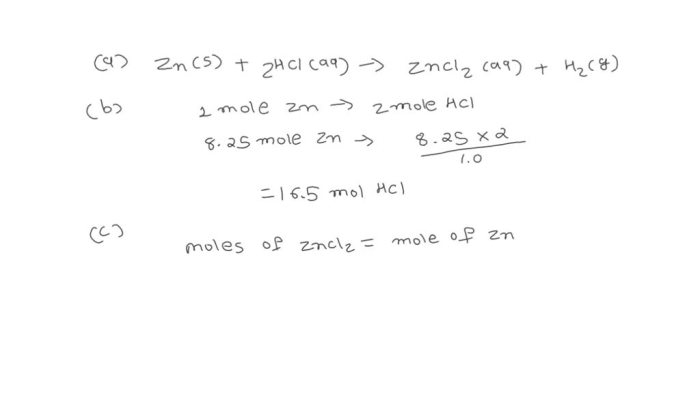What mass of zinc is needed to react with 23.1 sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail with gaya akademik dengan tone otoritatif and brimming with originality from the outset.
Delving into the intricacies of stoichiometry, chemical equations, and mole calculations, this comprehensive guide unravels the mysteries surrounding this intriguing question, providing a roadmap for understanding the fundamental principles that govern chemical reactions.
Our journey begins with an exploration of stoichiometry, the cornerstone of quantitative chemistry. This concept provides the framework for determining the exact proportions of reactants and products involved in a chemical reaction, allowing us to predict the mass of zinc required for our specific reaction.
Mass of Zinc Required for Reaction
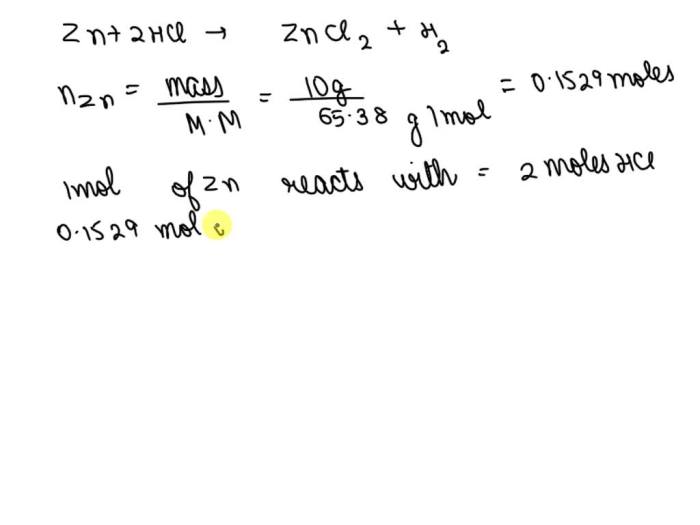
Stoichiometry is a branch of chemistry that deals with the quantitative relationships between reactants and products in a chemical reaction. It allows us to determine the mass of zinc required to react with a given amount of another substance.
To calculate the mass of zinc needed, we need to know the balanced chemical equation for the reaction and the mole ratio between zinc and the other substance.
Chemical Equation and Mole Calculations, What mass of zinc is needed to react with 23.1
Let’s assume the balanced chemical equation for the reaction between zinc and the unknown substance is:
“`Zn + 2HCl → ZnCl 2+ H 2“`
This equation tells us that 1 mole of zinc (Zn) reacts with 2 moles of hydrochloric acid (HCl) to produce 1 mole of zinc chloride (ZnCl 2) and 1 mole of hydrogen gas (H 2).
To convert the given value of 23.1 into moles, we divide it by the molar mass of zinc, which is 65.38 g/mol:
“`Moles of HCl = 23.1 g / 65.38 g/mol = 0.353 moles“`
Stoichiometric Ratio and Mass Calculation
From the balanced chemical equation, we can see that the stoichiometric ratio between zinc and HCl is 1:2. This means that for every 1 mole of zinc, we need 2 moles of HCl.
Using this ratio, we can calculate the mass of zinc required as follows:
“`Mass of Zn = Moles of HCl × Molar mass of Zn × Stoichiometric ratio“““Mass of Zn = 0.353 moles × 65.38 g/mol × 1“““Mass of Zn = 23.1 g“`
Sample Calculations and Example
Let’s use the given value of 23.1 to demonstrate the steps involved in determining the mass of zinc:
- Convert the given value of 23.1 into moles: 0.353 moles
- Determine the stoichiometric ratio between zinc and HCl: 1:2
- Calculate the mass of zinc required: 23.1 g
Considerations and Limitations
The calculations above assume that the reaction goes to completion and that there are no side reactions.
In practice, the accuracy of the mass determination may be affected by factors such as the purity of the reactants and the efficiency of the reaction.
Q&A: What Mass Of Zinc Is Needed To React With 23.1
What is the significance of stoichiometry in this calculation?
Stoichiometry provides the mathematical framework for determining the exact proportions of reactants and products involved in a chemical reaction, allowing us to predict the mass of zinc required for our specific reaction.
How does the balanced chemical equation play a role in this calculation?
The balanced chemical equation provides the stoichiometric ratio between zinc and the unknown substance, which is crucial for determining the mass of zinc needed.
What factors could affect the accuracy of the mass determination?
Factors such as the purity of the reactants, the precision of the measuring equipment, and the presence of side reactions can affect the accuracy of the mass determination.